What Is The Best Way To Increase Muscle Strength?
 The process of aging affects your body in many different ways, including the gradual loss of muscle mass and strength. It starts as early as age 30 and amounts to about 1% loss per year. In addition some people have a condition called sarcopenia where their muscles become weak more quickly than others after they reach an older age.
The process of aging affects your body in many different ways, including the gradual loss of muscle mass and strength. It starts as early as age 30 and amounts to about 1% loss per year. In addition some people have a condition called sarcopenia where their muscles become weak more quickly than others after they reach an older age.
Resistance exercise using barbells, dumb bells, machines, or your own weight (push ups, chin ups, etc) increases strength. This can help make up for the loss that occurs with aging which can be significant by the age of 70 or older. Also it is a whole lot easier to maintain strength than try to regain it later on. This research study explored whether just a small amount of activity daily is more helpful than a lengthier exercise workout less often.
The study involved four weeks of training that consisted of 3 groups performing an exercise that consisted of arm resistance. Two of the groups performed 30 contractions each week, with one group doing six contractions per day for a period of five days per week (6×5), while the other group crammed all 30 into a single day (30×1). Another group performed just six contractions one day per week.
The exercise consisted of “maximal voluntary eccentric bicep contractions” that were performed on a machine that measures muscle strength in each contraction of the muscle that you would do at a gym. An eccentric contraction is when the muscle lengthens and in this case like lowering a heavy dumbbell in a bicep curl.
Changes in muscle strength and thickness were measured and compared. The researchers found that over four weeks, people who did six contractions per day had greater increases than those doing 30 in both muscle thickness and strength. The former also saw their power output increase while performing this exercise; they could lift heavier weights after only five days of training!
The group that did 30 contractions in one day showed no increase in muscle strength, but they did see an increase of 5.8% for thickness. The 6×5 exercise plan showed significant increases in muscle strength, more than 10% with an increase of thickness similar to the 30X1 group. The group that did the six contractions once a week did not show any changes in muscle thickness or strength.
The finding is important because it suggests that short, regular sessions of exercise can produce similar results to those seen after longer ones. The current thought is that you have to do lengthy sessions at the gym, noted Ken Nosaka from ECU’s College Of Health And Human Sciences, but this isn’t always necessary. Just lowering heavy dumbbells gently for 1-6 times per day will provide enough physical stimulation for your muscles without overdoing things and possibly doing more harm than good.
The bicep curl is a common exercise for weightlifters and bodybuilders, but recent research suggests it’s not the only muscle group you need to work out. While the research required participants to perform the maximum effort with this particular move early findings indicate similar results can be achieved without needing as hard or pushing up against limits.
The body seems to respond better when exercises involve eccentric contractions at smaller doses rather than bigger loads less frequently. There is still much research left to be done on why this happens, but one theory is that it may relate to how often our brain is asked to make muscles perform in a particular manner.
If we want to be effective in our fitness goals, it’s important for us not only to focus on the weekly minute goal but also make exercise a daily activity. If a person exercises once every week or ten days, the results will be different from someone who does their workout five times per week. This research together with previous studies suggests how crucial accumulating small amounts of physical activity throughout each day can become over time rather than spending hours working out all at one time.
To view the original scientific study click below:
Greater effects by performing a small number of eccentric contractions daily than a larger number of them once a week



 Scientists have made a groundbreaking discovery about the mechanisms of healthy development in embryos, which could change our understanding on what we inherit from parents and how they shape us. The new research suggests that mothers may be passing on more of their DNA than we thought, with an epigenetic legacy for future generations.
Scientists have made a groundbreaking discovery about the mechanisms of healthy development in embryos, which could change our understanding on what we inherit from parents and how they shape us. The new research suggests that mothers may be passing on more of their DNA than we thought, with an epigenetic legacy for future generations.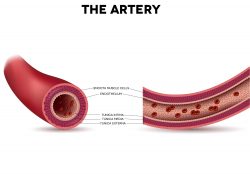 From the early 1900’s blood banks have been crucial in medical care. A team is now wanting to take the concepts a step further by utilizing stem cells in creating new arteries for people that have cardiovascular diseases. They have discovered a drug that might help decrease complications in people who undergo bypass surgery.
From the early 1900’s blood banks have been crucial in medical care. A team is now wanting to take the concepts a step further by utilizing stem cells in creating new arteries for people that have cardiovascular diseases. They have discovered a drug that might help decrease complications in people who undergo bypass surgery.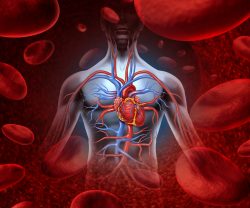 A new study is trying to understand early heart disease and development in relation to the mechanisms that determine cell fate. PSCs, or human pluripotent stem cells can conceivably produce a tissue the human body needs for repair. But developing technology for this to happen for a specialized cell, for example a beating heart muscle cell, has required intricate knowledge of developmental pathways and regulatory factors.
A new study is trying to understand early heart disease and development in relation to the mechanisms that determine cell fate. PSCs, or human pluripotent stem cells can conceivably produce a tissue the human body needs for repair. But developing technology for this to happen for a specialized cell, for example a beating heart muscle cell, has required intricate knowledge of developmental pathways and regulatory factors. It is known that the intestinal lining needs to regenerate daily to be a powerful barrier to counter pathogens while allowing nutrients to be absorbed. The responsibility for this comes from the intestine’s stem cells. They need to meet a level of constant replenishment and repair. But, for this to happen, the stem cell needs to decide if the conditions of the intestine are receptive. If the stem cell makes the wrong decision or coordinates it poorly, intestinal cancer or diseases could occur.
It is known that the intestinal lining needs to regenerate daily to be a powerful barrier to counter pathogens while allowing nutrients to be absorbed. The responsibility for this comes from the intestine’s stem cells. They need to meet a level of constant replenishment and repair. But, for this to happen, the stem cell needs to decide if the conditions of the intestine are receptive. If the stem cell makes the wrong decision or coordinates it poorly, intestinal cancer or diseases could occur. The indoor environment is usually more dangerous than the outdoors, with 90% of people’s time spent indoors. Chemicals from a variety of sources such as outdoor pollutants can seep into your home and cause problems for you there too! When it comes to health risks our bodies are constantly being bombarded by new chemicals – some good (like oxygen), others bad such as viruses or bacteria which want nothing better then an opportunity to live off of us. You might be surprised to learn that we are extremely harmful traveling emission sources of chemicals, including those found in our skin and breath.
The indoor environment is usually more dangerous than the outdoors, with 90% of people’s time spent indoors. Chemicals from a variety of sources such as outdoor pollutants can seep into your home and cause problems for you there too! When it comes to health risks our bodies are constantly being bombarded by new chemicals – some good (like oxygen), others bad such as viruses or bacteria which want nothing better then an opportunity to live off of us. You might be surprised to learn that we are extremely harmful traveling emission sources of chemicals, including those found in our skin and breath. It has been found from reviewing 49 studies from 1800 weightlifters that increasing protein intake by double the amount increased strength by 9% and added about 1 lb of muscle growth. The participants worked out for 6 weeks and lifted weights at least twice per week.
It has been found from reviewing 49 studies from 1800 weightlifters that increasing protein intake by double the amount increased strength by 9% and added about 1 lb of muscle growth. The participants worked out for 6 weeks and lifted weights at least twice per week.  New research has identified how cells communicate to repair muscle damage. When a muscle is damaged, stem cells and immune cells work together to repair the damage. But how these cells interact to complete the removal process of dead tissue and make new muscle fibers had been a mystery. Scientists have discovered that hyaluranic acid is the essential molecule that contributes to this interaction.
New research has identified how cells communicate to repair muscle damage. When a muscle is damaged, stem cells and immune cells work together to repair the damage. But how these cells interact to complete the removal process of dead tissue and make new muscle fibers had been a mystery. Scientists have discovered that hyaluranic acid is the essential molecule that contributes to this interaction.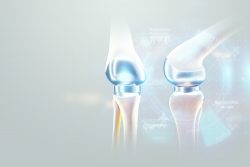 A new study has found a way to program stem cells in order to grow new cartilage on a 3-D template of the hip joint ball. This cartilage releases anti-inflammatory molecules that assist in fending off new arthritis occurrences. The new technology could supply an alternative to traditional hip replacement surgery and could remove the need for surgery for joint replacement surgery in some people.
A new study has found a way to program stem cells in order to grow new cartilage on a 3-D template of the hip joint ball. This cartilage releases anti-inflammatory molecules that assist in fending off new arthritis occurrences. The new technology could supply an alternative to traditional hip replacement surgery and could remove the need for surgery for joint replacement surgery in some people.  Hair follicle cells divide and die. But a new study has discovered a single chemical called TGF-beta that determines when this happens. It could ultimately treat baldness and may speed wound healing. Since follicles are a stem cell source they have the unique capability to be able to turn into other types of cells. This stem cell adaptability creates a path for repair of tissues and organs that have been damaged.
Hair follicle cells divide and die. But a new study has discovered a single chemical called TGF-beta that determines when this happens. It could ultimately treat baldness and may speed wound healing. Since follicles are a stem cell source they have the unique capability to be able to turn into other types of cells. This stem cell adaptability creates a path for repair of tissues and organs that have been damaged.