Plant-Based ALA Can Benefit Heart Health
 Heart disease is a leading cause of death in the United States, so it’s important to know which foods can help reduce the risk. A new research review has found that the major plant-based version of the nutrient omega-3 fatty acid, alpha-linolenic acid (ALA), can benefit heart health and reduce the risk of heart disease.
Heart disease is a leading cause of death in the United States, so it’s important to know which foods can help reduce the risk. A new research review has found that the major plant-based version of the nutrient omega-3 fatty acid, alpha-linolenic acid (ALA), can benefit heart health and reduce the risk of heart disease.
ALA is found in foods such as flaxseeds and walnuts and was linked to a 20% reduction in risk for heart disease and a 10% reduction in cardiovascular disease. There are many ways for people to get the recommended amount of omega-3 fatty acids which help promote overall health. One way is by eating seafood, but some people may not want to do this for various reasons. Even if someone eats seafood, incorporating plant-based ALA into their diet can provide extra benefits.
Recent research suggests that consuming ALA can be beneficial for heart health, particularly in those with low levels of omega-3s in their diet. However, this finding was also seen in people who had high levels of omega-3s from other sources. This suggests that ALA works synergistically with other omega-3s to promote heart health. Omega-3s have been linked with a lower risk of heart disease in the past, and this conclusion was based on evidence from marine-derived omega-3s. However, the benefits of ALA have been less well documented. By consuming ALA, you may be able to enjoy the benefits of omega-3s for heart health.
The review found that ALA can improve heart health by reducing the risk of heart disease and improving blood pressure and inflammation levels. The data analyzed came from both controlled trials and observational studies. Some of the observational studies relied on participants self-reporting their ALA intake, while others used biomarkers to measure levels of ALA in the blood. This provides a more accurate measure of ALA intake. Overall, the findings showed that ALA has a positive impact on heart health.
It is now more important to identify people who can receive the most benefit from eating ALA rich foods. The researchers found that ALA had beneficial effects on reducing lipoproteins and atherogenic lipids. This could help improve heart health by reducing cholesterol, low density-lipoprotein cholesterol, and triglycerides levels. Additionally, ALA was also able to lower blood pressure and inflammation levels.
From the research they were able to find supporting evidence that ALA can provide 0.6%-1% of the total day’s energy. This is close to 1.1 grams for women and 1.6 grams for men on a daily basis. This is equal to almost 1/2 oz of walnuts or just shy of 1 tsp of flaxseed oil.
In the future, more studies will be needed to assess the total effects of ALA on various chronic diseases. Additionally, further evaluation is needed on whether the current scientific articles support new and maybe higher dietary recommendations for ALA.
To view the original scientific study click below:
Impact of a-Linolenic Acid, the Vegetable w-3 Fatty Acid, on Cardiovascular Disease and Cognition



 Recent research has found that mice with high blood pressure lose more bone than those without the condition. As humans age, their bones become weaker and more brittle as a result of chronic inflammation which can lead to osteoporosis-related fractures when not treated properly. The team suggests treatments for this type of hypertension throughout early adulthood might help prevent further damage during later years. However, they also say it’s important we find out how prevalent these traits are among younger individuals so treatment options exist if needed.
Recent research has found that mice with high blood pressure lose more bone than those without the condition. As humans age, their bones become weaker and more brittle as a result of chronic inflammation which can lead to osteoporosis-related fractures when not treated properly. The team suggests treatments for this type of hypertension throughout early adulthood might help prevent further damage during later years. However, they also say it’s important we find out how prevalent these traits are among younger individuals so treatment options exist if needed. Getting more than the minimum recommended amount of exercise could help you live a healthier and longer life according to a new study. The current guidelines recommend 75 to 300 minutes of exercise on a weekly basis to benefit health. By doing more, you are linked to even a lower risk of issues with heart health and other risk factors. Up to 5 hours of exercise that is vigorous and 10 hours of moderate exercise such as walking may help decrease the risk of early death.
Getting more than the minimum recommended amount of exercise could help you live a healthier and longer life according to a new study. The current guidelines recommend 75 to 300 minutes of exercise on a weekly basis to benefit health. By doing more, you are linked to even a lower risk of issues with heart health and other risk factors. Up to 5 hours of exercise that is vigorous and 10 hours of moderate exercise such as walking may help decrease the risk of early death. A new study by researchers at the Univ. of Pittsburgh has found that people who are consistently active throughout the day tend to be happier and their performance on cognitive tests are better than those who have an irregular activity pattern. The findings suggest that patterns of activity, not just the intensity of the activity, are critical for healthy mental health and aging.
A new study by researchers at the Univ. of Pittsburgh has found that people who are consistently active throughout the day tend to be happier and their performance on cognitive tests are better than those who have an irregular activity pattern. The findings suggest that patterns of activity, not just the intensity of the activity, are critical for healthy mental health and aging. The impact of insufficient sleep on our health is largely unknown. However, a new study has found that people who consistently lose up to 1 1/2 hours per night are at increased risk for immune disorders such as cardiovascular disease or inflammatory conditions like arthritis.
The impact of insufficient sleep on our health is largely unknown. However, a new study has found that people who consistently lose up to 1 1/2 hours per night are at increased risk for immune disorders such as cardiovascular disease or inflammatory conditions like arthritis. A recent study has asked whether there are links between a person being physically active or being sedentary can be associated with a higher risk of death. The question became – does the risk change at all if a person is genetically predisposed to live a longer life? Earlier research has indicated that low physical activity and more time spent sitting are linked with a higher risk of death.
A recent study has asked whether there are links between a person being physically active or being sedentary can be associated with a higher risk of death. The question became – does the risk change at all if a person is genetically predisposed to live a longer life? Earlier research has indicated that low physical activity and more time spent sitting are linked with a higher risk of death.  A new study has developed a novel method for studying age related disorders of the brain. The team has focused on the neurodegenerative disorder Huntington’s Disease for the research.
A new study has developed a novel method for studying age related disorders of the brain. The team has focused on the neurodegenerative disorder Huntington’s Disease for the research. The process of aging affects your body in many different ways, including the gradual loss of muscle mass and strength. It starts as early as age 30 and amounts to about 1% loss per year. In addition some people have a condition called sarcopenia where their muscles become weak more quickly than others after they reach an older age.
The process of aging affects your body in many different ways, including the gradual loss of muscle mass and strength. It starts as early as age 30 and amounts to about 1% loss per year. In addition some people have a condition called sarcopenia where their muscles become weak more quickly than others after they reach an older age. Scientists have made a groundbreaking discovery about the mechanisms of healthy development in embryos, which could change our understanding on what we inherit from parents and how they shape us. The new research suggests that mothers may be passing on more of their DNA than we thought, with an epigenetic legacy for future generations.
Scientists have made a groundbreaking discovery about the mechanisms of healthy development in embryos, which could change our understanding on what we inherit from parents and how they shape us. The new research suggests that mothers may be passing on more of their DNA than we thought, with an epigenetic legacy for future generations.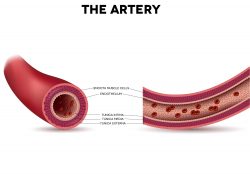 From the early 1900’s blood banks have been crucial in medical care. A team is now wanting to take the concepts a step further by utilizing stem cells in creating new arteries for people that have cardiovascular diseases. They have discovered a drug that might help decrease complications in people who undergo bypass surgery.
From the early 1900’s blood banks have been crucial in medical care. A team is now wanting to take the concepts a step further by utilizing stem cells in creating new arteries for people that have cardiovascular diseases. They have discovered a drug that might help decrease complications in people who undergo bypass surgery.