Changing Your Sleep Pattern Could Help Depression
 If you suffer from depression, a new study just might help you. A person could reduce their risk of having major depression by 23% by adjusting their sleep schedule according to a genetic study published May 26, 2021. The study consisted of 840,000 participants and was conducted by the Univ. of Colorado at Boulder and the Broad Institute of MIT and Harvard. It’s one of the first studies to signify what can influence mental health based on how much or how little change is required.
If you suffer from depression, a new study just might help you. A person could reduce their risk of having major depression by 23% by adjusting their sleep schedule according to a genetic study published May 26, 2021. The study consisted of 840,000 participants and was conducted by the Univ. of Colorado at Boulder and the Broad Institute of MIT and Harvard. It’s one of the first studies to signify what can influence mental health based on how much or how little change is required.
Researchers have often wondered how the relationship between mood and sleep timing has on a person. This study reveals strong evidence that a person’s certain sleep time influences their mood. Just going to sleep an hour earlier than usual, a person’s risk of depression is significantly lowered.
From previous studies it has been learned that early risers are less likely to suffer from depression than night owls. But it has been hard to decipher what causes it since mood disorders can disrupt normal sleep patterns. So to find out, data from a DNA testing company was used. A method called “Mendelian randomization” helped decipher genetic associations as to cause and effect.
Of the most common genetic variants, more than 340 were used that could affect a person’s chronotype or their behavior to sleep at a certain time. 850,000 participants were assessed their de-identified genetic data and the researchers also had 85,000 people wear a sleep tracker for 7 days. The study included 250,000 people that filled out a sleep preference questionnaire. This was to give them a more finite picture, even down to the hour of how the gene variants influence sleep and wake up times a person has.
The participants were divided into three groups. One-third identified themselves as morning larks. The second group, the night owls, were about 9% and all of the rest were somewhere in the middle. The researchers took into consideration a person’s genetic information, their diagnosis of any mental disorders, and medical and prescription records.
The data suggests that those people that have genetic variants that predispose them to wake up early do have a lower risk of depression. If you are already an early riser, it was unclear if a person could benefit from waking up earlier. Because early risers have a greater amount of daylight exposure, the research suggests this could benefit their hormones and influence mood.
If a person goes to sleep even one hour earlier it correlated with a lower risk of depression by 23%. If they would go to sleep two hours earlier and slept the same amount they could cut it by 40%. So people that normally stay up late in the evening could benefit from an earlier bedtime. So if you can shift to an earlier sleep pattern, it may be beneficial and possibly lower your risk of depression.
To view the original scientific study click below:
Genetically Proxied Diurnal Preference, Sleep Timing, and Risk of Major Depressive Disorder



 Tai Chi, a Chinese martial art and system of calisthenics is now being compared to conventional exercise. In a recent study from the University of Hong Kong, the Chinese University of Hong Kong; Chinese Academy of Sciences; and UCLA it shows that Tai Chi can duplicate the benefits of exercise. It can reduce the waist circumference in middle to older adults (over 50 years of age) that have obesity in the central region of the body.
Tai Chi, a Chinese martial art and system of calisthenics is now being compared to conventional exercise. In a recent study from the University of Hong Kong, the Chinese University of Hong Kong; Chinese Academy of Sciences; and UCLA it shows that Tai Chi can duplicate the benefits of exercise. It can reduce the waist circumference in middle to older adults (over 50 years of age) that have obesity in the central region of the body. In the recent study at the Salk Institute and published by Nature Communications, researchers showed that by using molecular compounds it can speed up the regeneration of muscle tissue. These compounds are commonly used in the research of stem cells. As we age loss of muscle mass is a concern in regards to causing disabilities.
In the recent study at the Salk Institute and published by Nature Communications, researchers showed that by using molecular compounds it can speed up the regeneration of muscle tissue. These compounds are commonly used in the research of stem cells. As we age loss of muscle mass is a concern in regards to causing disabilities. Spring is a great time to eat berries now that they are in season, especially strawberries. Eating berries offers many health benefits and a wide range of nutrients. They have a low glycemic index and are lower in sugar than most other fruits. They are also considered a heart-healthy food. The bright colors of berries come from polyphenols. Those are antioxidants which are far more powerful than vitamin C and other common antioxidants found in supplements. The brightness and different colors of berries reflect different kinds and amounts of polyphenols. Consuming many different kinds of polyphenols is beneficial so it is good to eat different varieties of berries.
Spring is a great time to eat berries now that they are in season, especially strawberries. Eating berries offers many health benefits and a wide range of nutrients. They have a low glycemic index and are lower in sugar than most other fruits. They are also considered a heart-healthy food. The bright colors of berries come from polyphenols. Those are antioxidants which are far more powerful than vitamin C and other common antioxidants found in supplements. The brightness and different colors of berries reflect different kinds and amounts of polyphenols. Consuming many different kinds of polyphenols is beneficial so it is good to eat different varieties of berries.  A team at Utah State University have shown that using silkworm silk for growing skeletal muscle cells will improve upon typical methods of cell culture. The hope is that this will lead to better treatments for muscles that have atrophied.
A team at Utah State University have shown that using silkworm silk for growing skeletal muscle cells will improve upon typical methods of cell culture. The hope is that this will lead to better treatments for muscles that have atrophied.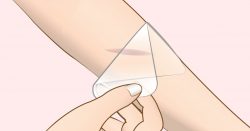 In a new development at Chalmers University of Technology, Sweden, researchers have designed a new material to prevent infections in wounds. The specially designed hydrogel consists of material that works against all kinds of bacteria even antiobiotic-resistant ones.
In a new development at Chalmers University of Technology, Sweden, researchers have designed a new material to prevent infections in wounds. The specially designed hydrogel consists of material that works against all kinds of bacteria even antiobiotic-resistant ones.  The Journal of Clinical Neurology and Neurosurgery has reported that researchers from Yale Univ. and Japan have intravenously injected patients with spinal cord injuries with bone marrow derived stem cells or MSCs from their own bodies.
The Journal of Clinical Neurology and Neurosurgery has reported that researchers from Yale Univ. and Japan have intravenously injected patients with spinal cord injuries with bone marrow derived stem cells or MSCs from their own bodies.  Salmon is said to have great health benefits. But, does it matter what kind of salmon you eat? Yes it does. Wild-caught salmon is by far better for you than farm-raised salmon. But why?
Salmon is said to have great health benefits. But, does it matter what kind of salmon you eat? Yes it does. Wild-caught salmon is by far better for you than farm-raised salmon. But why? Recent research led by Stanford University shows how increasing a persons access to nature is beneficial for overall health. It shows that people that live in cities need places to go to that will increase their physical activity. Places in nature such as parks, lakes, green spaces will help boost a persons physical activity, therefore, increasing their overall wellbeing.
Recent research led by Stanford University shows how increasing a persons access to nature is beneficial for overall health. It shows that people that live in cities need places to go to that will increase their physical activity. Places in nature such as parks, lakes, green spaces will help boost a persons physical activity, therefore, increasing their overall wellbeing. Green-leafy vegetables are among the very healthiest foods you can eat. They are packed with vitamins, minerals, fiber, polyphenols and more. Of course, they are also very low in calories making them a great food for losing or maintaining weight.
Green-leafy vegetables are among the very healthiest foods you can eat. They are packed with vitamins, minerals, fiber, polyphenols and more. Of course, they are also very low in calories making them a great food for losing or maintaining weight.